Use case: Determine total human thyroxine (T4)
Thyroxine (T4) is a commonly measured thyroid hormone for diagnosis of the thyroid function. Determining the total quantity of human thyroxine in serum is a standard use case for interpolation on a calibration curve.
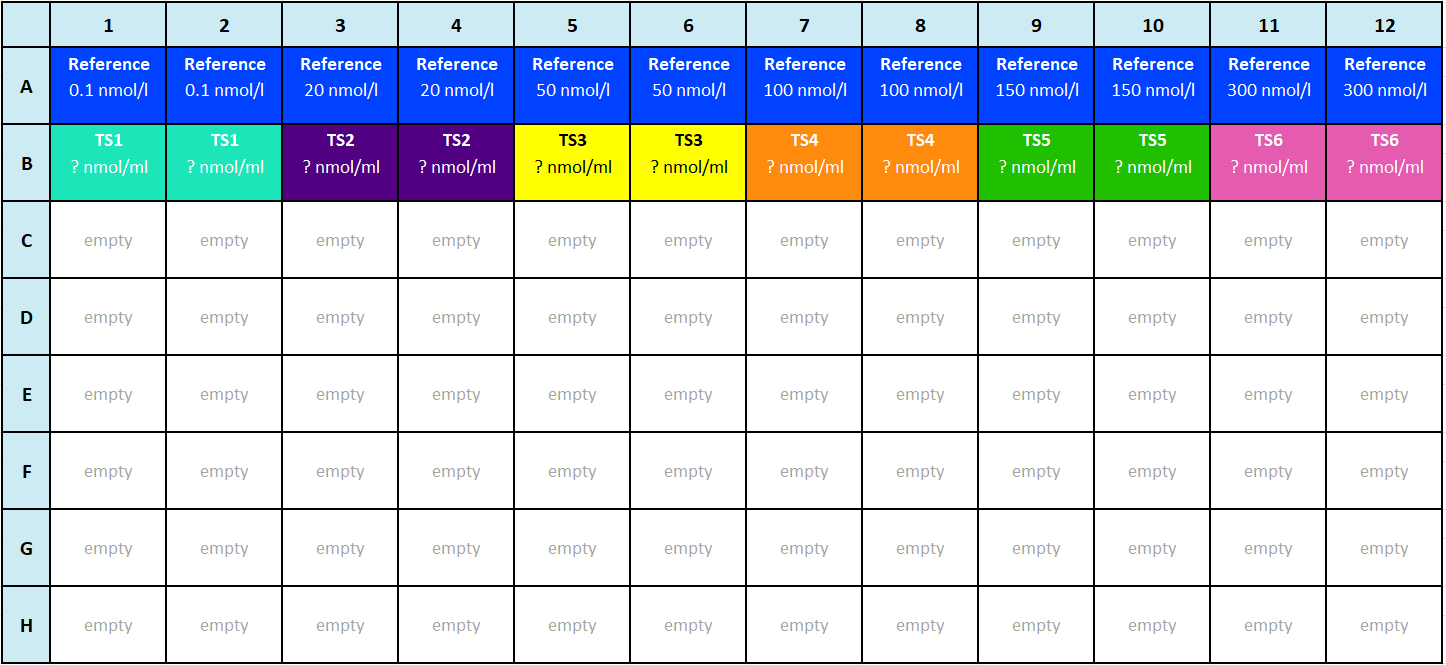
Plate layout
For this use case, you work with an assay with a 96-well plate layout. The assay has one Standard sample and six Test samples.
Steps

You begin by creating a new Dose-response analysis document. In the next steps, you use the Content editor to set up the assay elements for your samples and the preparation schemes, followed by the definition of the analytical settings. In steps five and six, you use the By Position editor to define your plate layout and enter your observation data. As the last step, you calculate the reportable values for your assay.
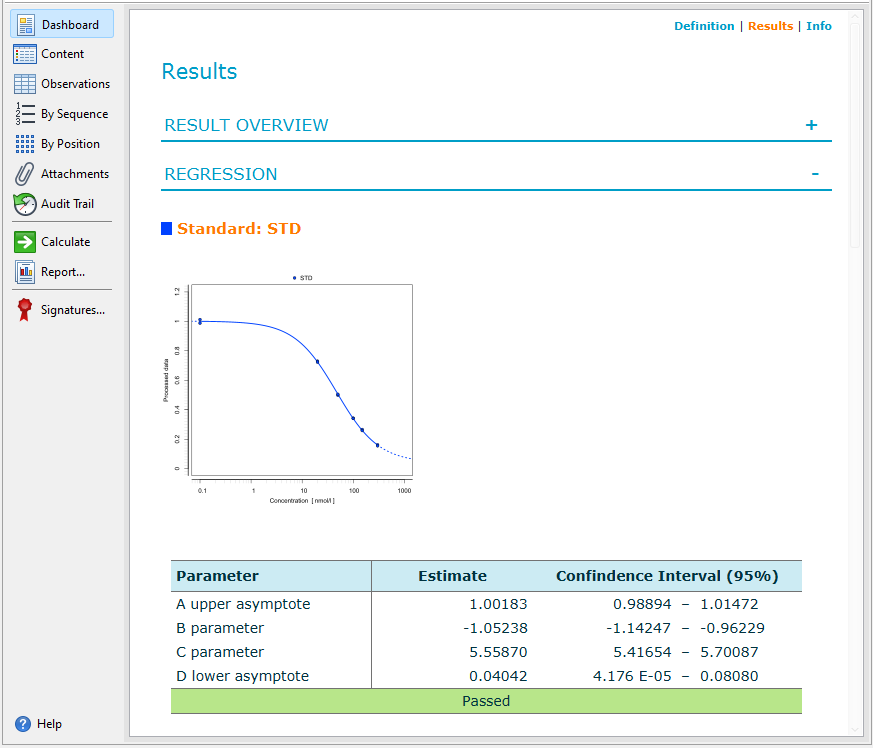
Results
Once calculated, you can access the document dashboard to view key document attributes, a graphical summary of the calculation results, and a representation of the plate layout you used for the assay.
To open the dashboard of a document, on the action bar, select ![]() Dashboard. For
details on how to work with the dashboard, see the Dashboard
topic.
Dashboard. For
details on how to work with the dashboard, see the Dashboard
topic.
Reporting
Create reports to download and share your assay results. PDF reports contain the data available on the dashboard, enriched with details like the database that holds the document, and detailed calculation results for each assay element. Use CSV reports to export your assay data for further processing.
To generate a report for a document, on the action bar, select ![]() Report. For details on how to work
with reports, see the Create reports topic.
Report. For details on how to work
with reports, see the Create reports topic.
