Equivalence margin development
Use Equivalence margin development documents to develop and verify test systems based on historic assays and apply these test systems in current runs of dose response assays.
About equivalence margin development
Equivalence margin development documents build on the idea of equivalence testing for calibration curves in accordance to USP <1032>. Equivalence tests assess whether curve characteristics, such as ANOVA terms and parameter estimates or their confidence intervals, fall within predefined acceptance limits.
While standard equivalence tests focus on evaluating results of individual assay runs, equivalence margin development lets you use historic assay runs to develop acceptance criteria (margins) that support you during assay evaluation.
Visualization and simulation tools let you explore and confirm how your test strategies perform with historic assay data. These insights ensure that your acceptance criteria are realistic and robust before applying them in productive environments.
About this document type
Equivalence margin development documents support you in analyzing historic assay data to define suitable equivalence margins for your assays. By evaluating past assay results, you can create test strategies and visualize their performance to verify whether they reliably distinguish acceptable from unacceptable assay outcomes.
Document structure
Each Equivalence margin development document has five default sections, that is, Settings, Datatable, Margin development, Test strategies, and Visualization, plus one or more optional Comment sections.
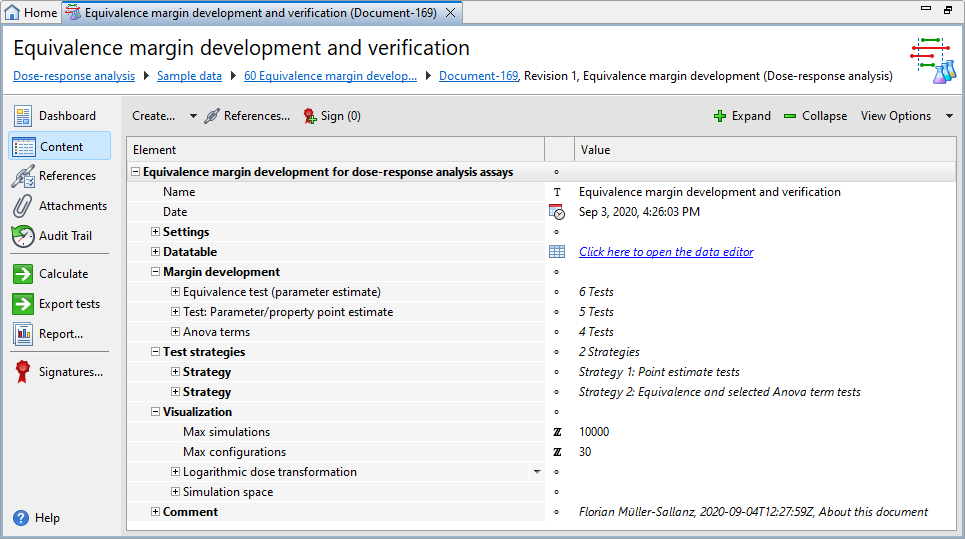
| Section | Description |
|---|---|
|
Settings |
Lets you select the calculation you require and set the confidence and tolerance levels. |
|
Datatable |
Lets you adjust the dataset structure used to calculate the margins. |
|
Margin development |
Lets you select the suitability tests for which you want to calculate margins. |
|
Test strategies |
Lets you define the test strategies you require for verification. |
|
Visualization |
Lets you set the parameters you require for simulation. |
|
Comment |
Add extra information to your assay. The PDF reports display the comments you add on the first page. You can use a simplified Wiki notation for the text. For details on the Wiki notation, see the Simplified Wiki notation topic. |
Get started
To get started efficiently, we recommend becoming familiar with the concepts that make up the document structure, as they form the foundation for a structured and reliable evaluation in PLA 3.0. For details and best practices, see the Concepts for efficient setup topic.
