Test system definition
Use Test system definition documents to define a collection of suitability tests and corresponding margins for dose response assays.
About this document type
Test system definition documents let you set up a centralized suitability test system for repeated use. Instead of defining tests within each Dose-response analysis document, you reference a shared Test system definition document to ensure consistency across your assays.
You can include one or more suitability tests in each Test system definition document and define their parameters, scope, and severity level. For details on these settings, see the Suitability tests topic.

To set up these references, you use the Content editor of your Dose-response analysis document. For details on the required steps, see the Create references to documents topic.
Document structure
Each Test system definition document has one default section, that is Suitability tests, plus one or more optional Comment sections. Use the subsections to add the assay and sample suitability tests you require.
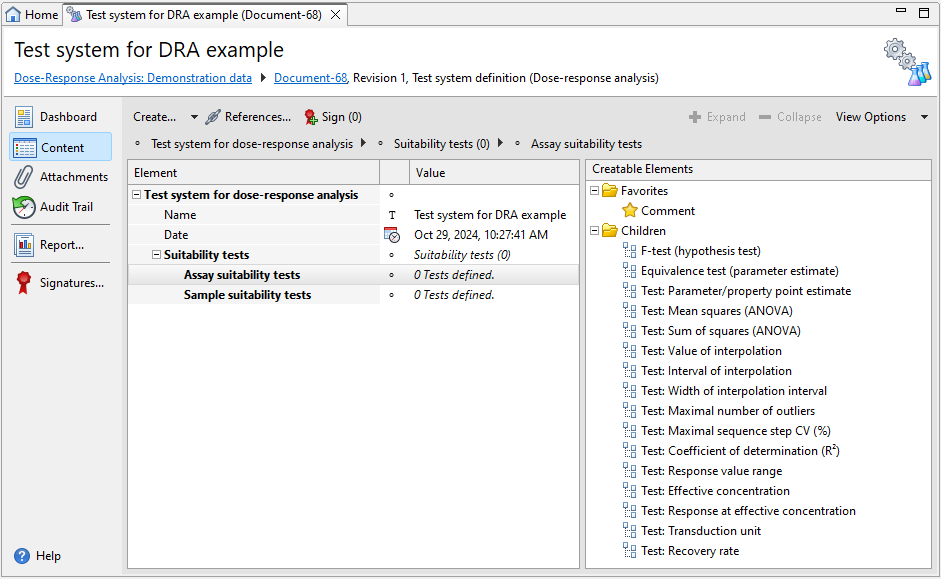
Suitability tests
Test system definition documents for dose-response analysis offer a variety of tests from the following groups. For details on each test, see the respective subtopic.
| Group | Includes |
|---|---|
|
ANOVA terms |
F-test (model, lack of fit), mean squares, sum of squares (both: model, residual error, lack of fit, pure error, total error) |
|
Equivalence tests |
Parameter estimates with their confidence intervals (nonlinear models: A, B, C, D, G, A-D, A/D; linear models: intercept, slope) |
|
Regression parameters |
Parameter estimates (nonlinear models: A, B, C, D, G, A-D, A/D, slope; linear models: intercept, slope) |
|
Observation data |
Number of outliers, sequence step CV, response value range |
|
Calculation results |
r2, interpolation (mean, interval, width), effective concentration (concentration, response), transduction unit, recovery rate |
