Export and reuse test strategies
Learn how to export the test strategies of an Equivalence margin development document as a Test system definition document.
About this task
Exporting test strategies as Test system definition documents lets you apply the developed tests outside of the Equivalence margin development document. This way, you can reuse your strategies for assay evaluation without repeating the development steps. The document names keep the link to their origin for traceability.
Tip:
Test system definition documents created by Equivalence margin development documents list all test strategies as sample
suitability tests by default. Before you begin working with the Test system definition document, make sure to move any tests you
intend to use as assay suitability tests to the appropriate section.
Procedure
To export test strategies:
-
On the action bar of your Equivalence margin development document, select
 Export
tests.
Results: PLA 3.0 creates one Test system definition document for each test strategy you defined. The name of each document is based on the name of the strategy, followed by the name of the Equivalence margin development document in parentheses.
Export
tests.
Results: PLA 3.0 creates one Test system definition document for each test strategy you defined. The name of each document is based on the name of the strategy, followed by the name of the Equivalence margin development document in parentheses.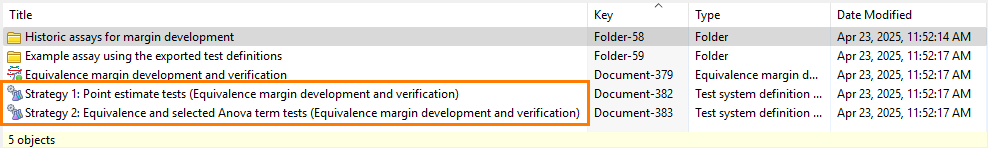
Figure 1. Test system definition documents created using test strategies - Save the Equivalence margin development document.
To reuse a test strategy:
- In the Content editor of your Dose-response analysis document, go to .
- Use the Creatable elements pane to add a Test system reference element.
-
In Test system reference element, select the
Value column, then select the browse icon
 .
.
- In the Document reference dialog, navigate to the Test system definition document that has the test strategy you want to use, select it, and then select OK.
- Save the Dose-response analysis document.
