Use case: Validate a cleaning process for pharmaceutical water
In the pharmaceutical industry, water is an important resource both in synthesizing and developing products and in cleaning vessels and other types of equipment. Validating the cleaning processes for pharmaceutical water to prevent product contamination is therefore crucial.
Overview
Different pharmaceutical uses require different grades of water. The European Pharmacopoeia provides quality standards for the grades of water listed below. To be deemed clean, the endotoxin level for both grades must not exceed the limit of 0,25 EU/ml.
-
Purified water (aqua purificata), that is, water that has been filtered or processed to remove impurities and make it suitable for use.
-
Water for Injections (WFI) (aqua ad iniectabile), that is, water used for dissolving and diluting medicinal products, which will be given as injection by syringe or infusion.
For validation, a third grade of water is used, that is, endotoxin-free water. This grade is considered to be cell culture grade as it is sterile filtered through a 0.05-micron filter to be free of endotoxins.
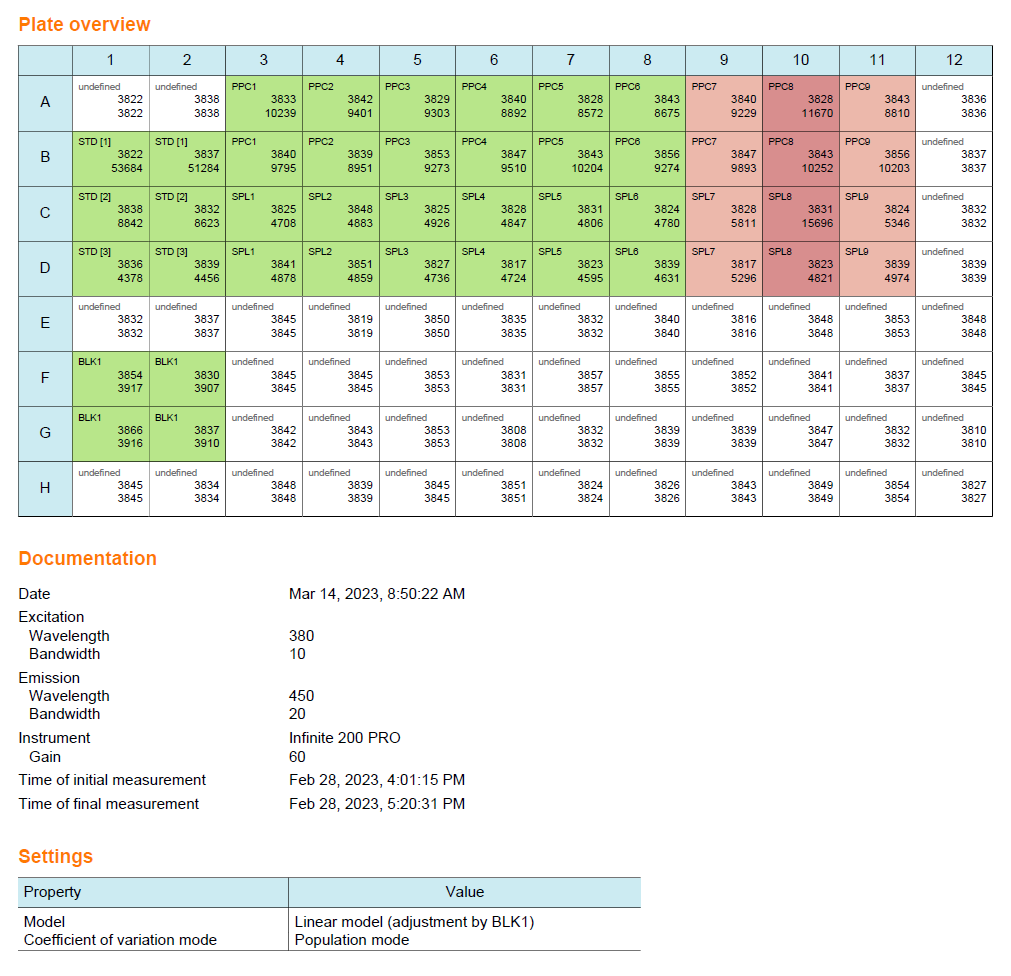
Plate layout
For this use case, you work with an assay with a 96-well plate layout. The plate layout is based on the GOPLATE™ layout, prepared with the ENDOZYME® II GO assay kit. Some wells remain empty.
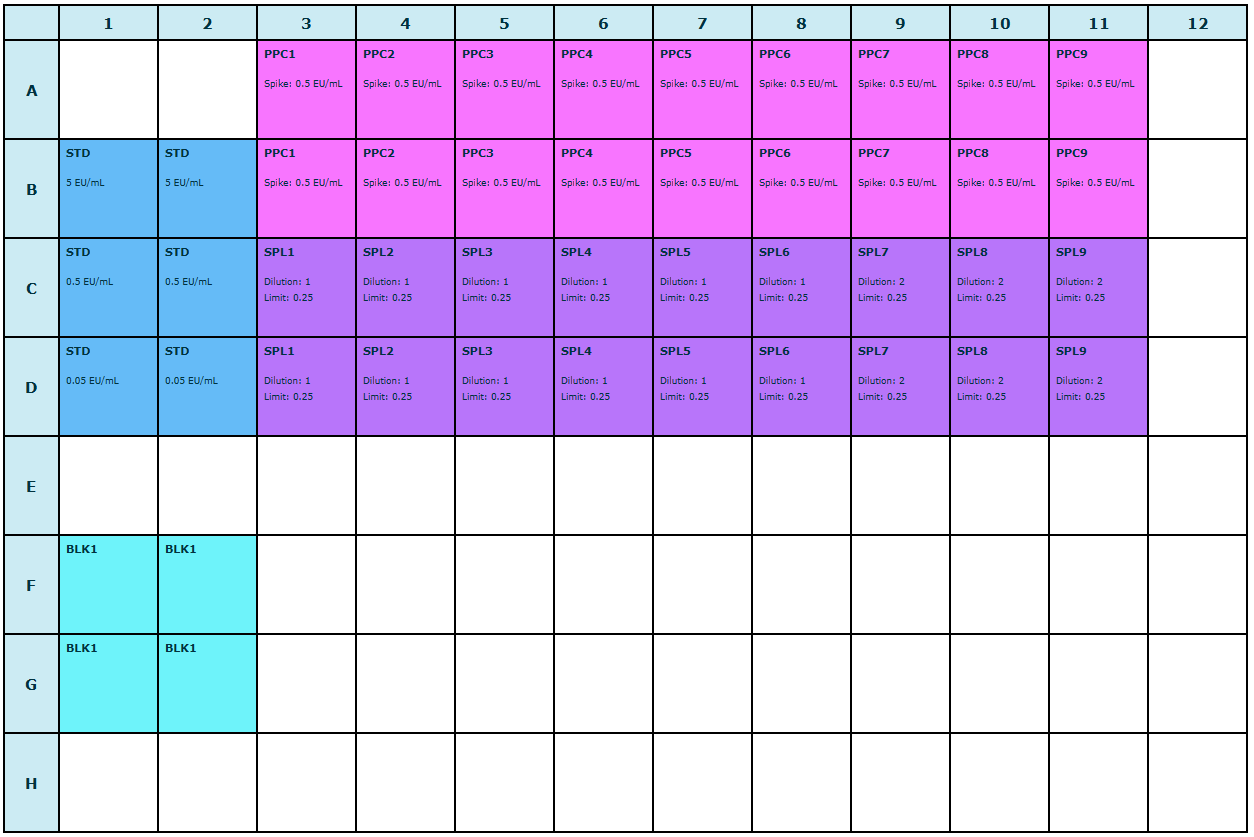
Steps

You begin by creating a new bioMérieux endotoxin detection assay document. In the next steps, you use the Content editor to set up the assay elements for your samples and define your analytical settings. In steps four and five, you use the By Position editor to define your plate layout and enter your observation data. As the last step, you calculate the reportable values for your assay.
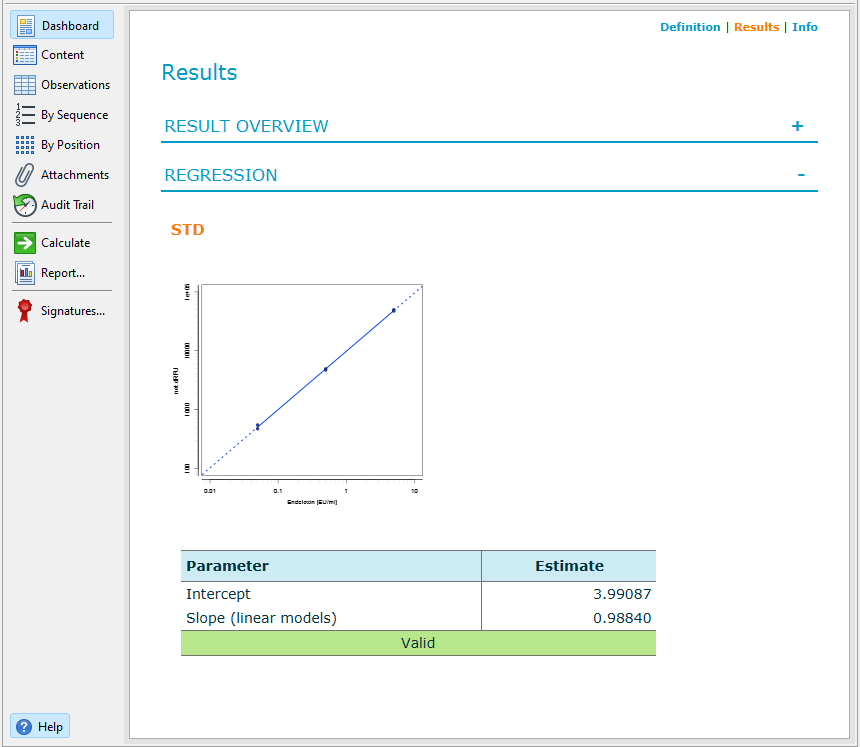
Results
Once calculated, you can access the document dashboard to view key document attributes, a graphical summary of the calculation results, and a representation of the plate layout you used for the assay.
To open the dashboard of a document, on the action bar, select ![]() Dashboard. For
details on how to work with the dashboard, see the Dashboard
topic.
Dashboard. For
details on how to work with the dashboard, see the Dashboard
topic.
Reporting
Create reports to download and share your assay results. PDF reports contain the data available on the dashboard, enriched with details like the database that holds the document, and detailed calculation results for each assay element. Use CSV reports to export your assay data for further processing.
To generate a report for a document, on the action bar, select ![]() Report. For details on how to work
with reports, see the Create reports topic.
Report. For details on how to work
with reports, see the Create reports topic.