bioMérieux endotoxin detection assays
Use bioMérieux endotoxin detection assay documents to detect or extrapolate endotoxin concentrations in potentially contaminated test substances by comparison with a defined endotoxin dilution series.
About this document type
bioMérieux assay kits use an optical, differential fluorescence measurement. An initial measurement, performed immediately after assay preparation, is subtracted from a final measurement, carried out after a defined incubation time. The delta value thus obtained for each well represents the actual response for this assay.
For the Standard and the Test samples, the final response value is greater than the initial response value and both values are non-negative. As values are obtained using fluorescence intensity measurement, the measurement unit involved is referred to as 'relative fluorescence unit' (RFU). After calculating the delta value, the bioMérieux endotoxin detection assay document type gives the assay-relevant response in the unit 'delta relative fluorescence units (dRFU).'
Document structure
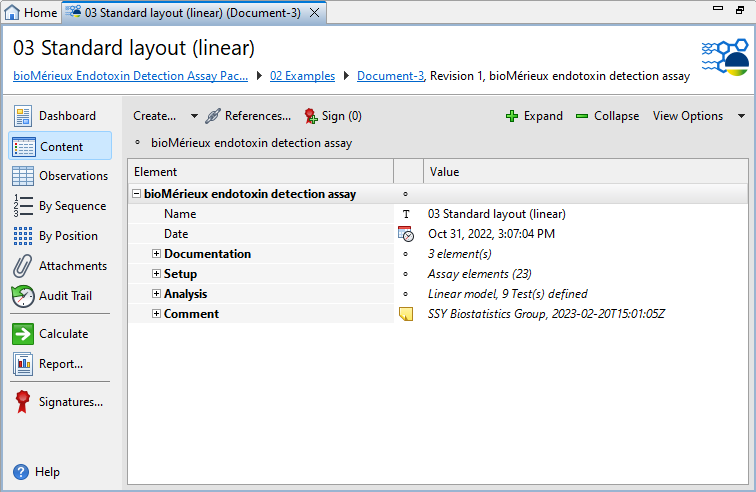
| Section | Description |
|---|---|
|
Documentation |
Provide supplemental information on the setup of your assay such as the operator, the incubation procedure, and the instrument gain. |
|
Setup |
Set up the assay and organize your observation data. For details on the available settings, see the Assay setup and Test system topics. |
|
Analysis |
Specify the regression model, Coefficient of variation mode, a gain optimization routine, and statistical tests to be used in calculations. For details on the available settings, see the Analytical settings topic. |
|
Comment |
Add extra information to your assay. The PDF reports display the comments you add on the first page. You can use a simplified Wiki notation for the text. For details on the Wiki notation, see the Simplified Wiki notation topic. |
