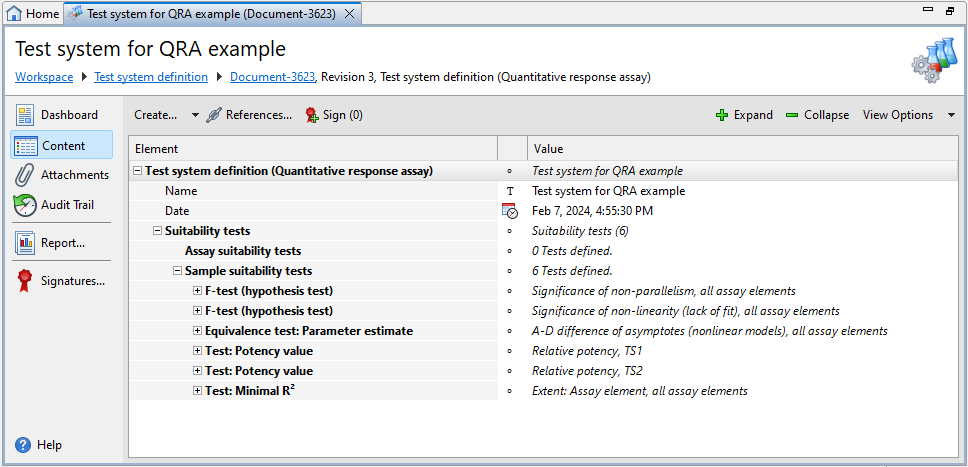
Test system definition
Use Test system definition documents of the Biological Assay Package to define a collection of suitability tests and corresponding margins for Quantitative response assay documents.
Tip:
You can identify documents of this type by the  icon in the upper right
corner of the Content editor.
icon in the upper right
corner of the Content editor.
Document structure
Each Test system definition document have one default section, that is Suitability tests, plus one or more optional Comment sections.
Use the subsections to add the test types you require, that is assay suitability tests, sample suitability tests, and document suitability tests.