Quantitative response assays
Use Quantitative response assay documents to calculate the potencies of test samples relative to an established standard. You can also use them to determine absolute potencies based on stock solutions or raw materials.
About this document type
Quantitative response assay documents cover all biological assay types that are based on quantitative response, that is, parallel-line, parallel logistic (full curve fits), and slope ratio assays.
They are often the first step in broader assay workflows, allowing you to structure and evaluate your data step by step, from defining samples and dilutions to assigning plate layouts and performing calculations. You can use their results as input for combination calculations or include them in automated workflows using protocols.
Document structure
Each Quantitative response assay document has three default sections, that is, Documentation, Setup, and Analysis, plus an optional Settings and one or more Comment sections.
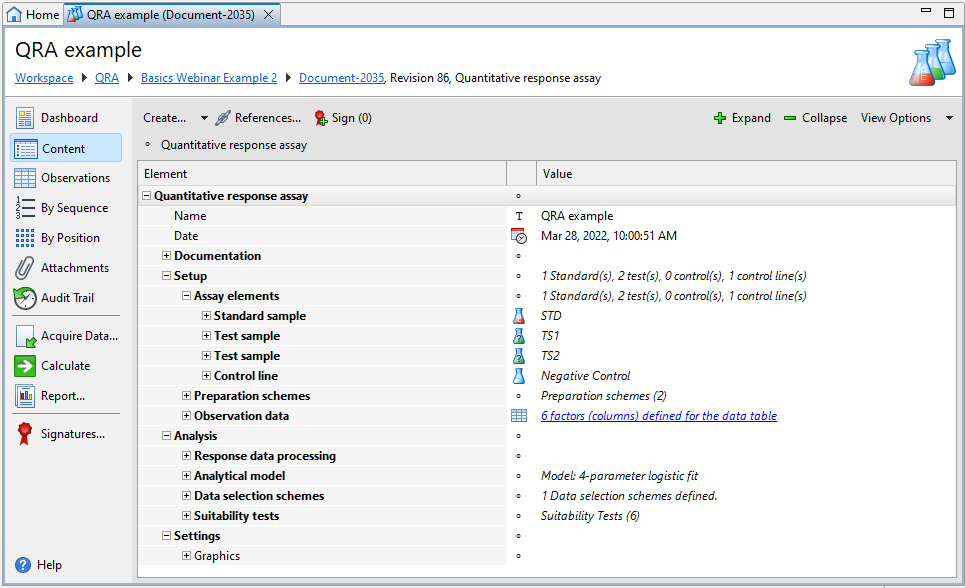
| Section | Description |
|---|---|
|
Documentation |
Provide supplemental information on the setup of your assay that is needed for documentary purposes but is not required for calculation or the organization of observation data. |
|
Setup |
Set up your assay and organize your observation data. For details on the available settings, see the Assay setup topic. |
|
Analysis |
Specify the processing of response data, the analytical model, data selection schemes, and suitability tests you want to use in calculations. For details on the available settings, see the Analytical settings and Suitability testing topics. |
|
Settings |
Define how you want to graphically display assay results. |
|
Comment |
Add extra information to your assay. The PDF reports display the comments you add on the first page. You can use a simplified Wiki notation for the text. For details on the Wiki notation, see the Simplified Wiki notation topic. |
