Equivalence margin development
Use Equivalence margin development documents to develop and verify test systems based on historic assays and apply these test systems in new development of quantitative response assays.
About this document type
You can develop equivalence margins for Quantitative response assay documents and Dose-response analysis documents. This topic covers Quantitative response assay documents.
Document structure
Each Equivalence margin development document has five default sections, that is, Settings, Data table, Margin development, Test strategies, and Visualization, plus one or more optional Comment sections.
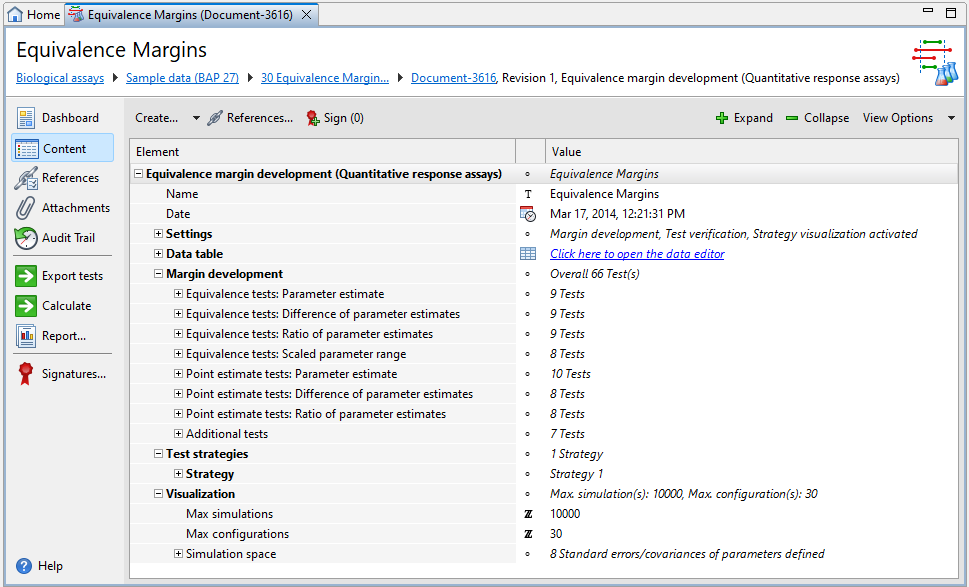
| Section | Description |
|---|---|
|
Settings |
Select the steps you require and set the confidence and tolerance levels. |
|
Data table |
Adjust the dataset structure used to calculate the margins. |
|
Margin development |
Select the tests you require to calculate margins. |
|
Test strategies |
Define the test strategies you require for verification. |
|
Visualization |
Set the parameters you require for simulation. |
|
Comment |
Add extra information to your assay. The PDF reports display the comments you add on the first page. You can use a simplified Wiki notation for the text. For details on the Wiki notation, see the Simplified Wiki notation topic. |
