Import assay data
Import response values created with the bioMérieux ENDOLISA®, ENDOZYME® II GO, and ENDOZYME® II GO STRIPS assay kits for further processing.
Before you begin
| Requirement | Background | Steps |
|---|---|---|
|
Add-on version 2.0.0 of the Tecan Magellan™ Import Module for bioMérieux Endotoxin Detection Assays Data Acquisition Module is activated in your database. |
All your add-ons are automatically made available in PLA 3.0. To use a Data Acquisition Module, you have to activate the corresponding add-on in your database. |
See the Install the import module topic. |
|
At least one endotoxin assay template is available in your database. |
The import module uses endotoxin assay templates provided as sample data by the bioMérieux Endotoxin Detection Assay Package. |
See the bioMérieux Endotoxin Detection Assay Package topic. |
|
Your Tecan Magellan™ reader control software is installed on the computer you want to use for the data import. |
To select workspace file pairs, a functional version of the Tecan Magellan™ reader control software is required. |
Consult the documentation of your Tecan Magellan™ software. |
About this task
You import measurements from Tecan Magellan™ reader control software based on workspace file pairs. During the import, you select the pairs containing the respective measurements and specify the template you want to use. For each selected pair, the import process creates a document based on the selected template.
PLA 3.0 imports the assay data with traceability information, details about the source file and the import module used. Where possible, traceability information from the workspace file (time stamp of the import, the operator who acquired the data, the reader and its serial number) is used.
Procedure
- In the Navigator, right-click the folder into which you want to import your assay data.
-
From the context menu, select
 Import.
Import.
-
In the Importing documents dialog, select
 'bioMérieux endotoxin detection assays,' and then
select OK.
Results: The Tecan Magellan™ Import Module for bioMérieux Endotoxin Assays dialog opens. The folder from which you started the import is set as the target folder.
'bioMérieux endotoxin detection assays,' and then
select OK.
Results: The Tecan Magellan™ Import Module for bioMérieux Endotoxin Assays dialog opens. The folder from which you started the import is set as the target folder. -
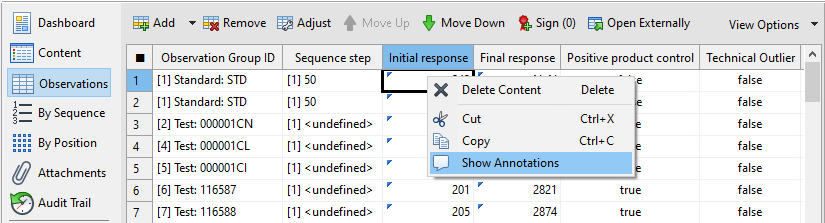
Under Configuration, next to Endotoxin assay
template, select …, and then select the
template you want to use for the import.
You can use the templates provided by the bioMérieux Endotoxin Detection Assay Package to import your data. You can also create your own templates or modify the preconfigured ones to match your requirements. For details on the preconfigured templates, see the Templates topic.
-
From the Sample ID matching strategy list box, select
whether the plate layout of the Tecan Magellan™ reader control software can
differ from the one in PLA 3.0.
- All samples: The plate layouts have to match, no differences are allowed. PLA 3.0 applies all sample IDs from your source data to the respective assay elements.
- Test samples only: The plate layouts have to match for Test samples only. Standard, Blank control, and PPC control can differ. PLA 3.0 applies the sample IDs from the test samples to the respective Test sample assay elements.
- No matching: The plate layouts do not have to match. PLA 3.0 does not apply any sample ID.
-
Use the Import dilution factors option to select which
dilution factor setup you want to use.
- Deactivate the option to use the dilution factor setup of the template.
- Activate the option to use the dilution factor setup of your source data.
-
Under Magellan™ workspaces, select the workspace file
pairs you want to import.
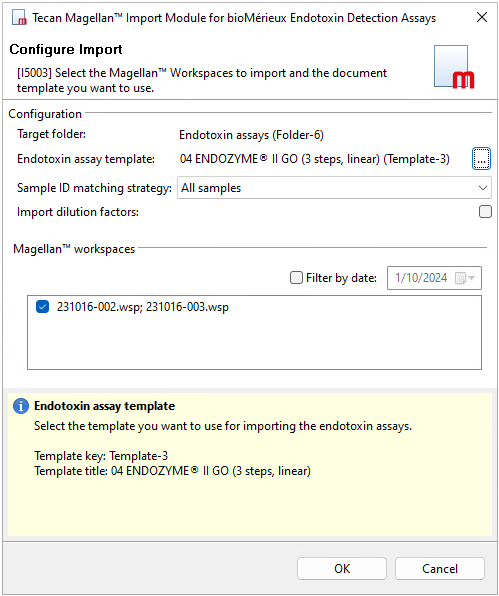
Figure 1. Importing Tecan Magellan™ workspace files into PLA 3.0 Tip:Use the Filter by date option to reduce the list of displayed workspace files. - Confirm your selection with OK.
Results
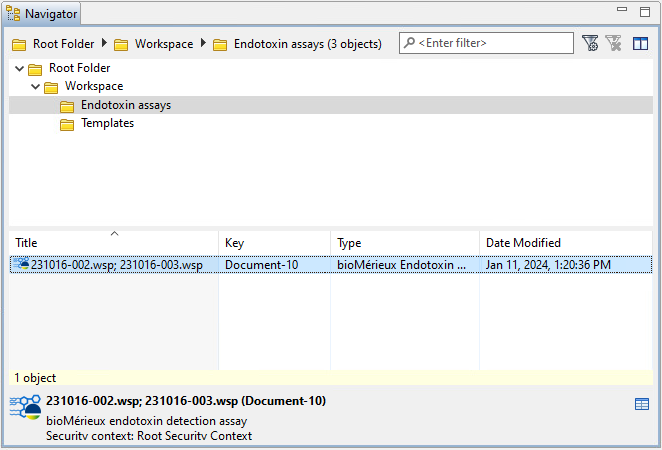
In the Observations editor, PLA 3.0 displays details about the process as an
annotation to the imported value. You can identify an annotated
value by a small blue triangle in the upper left corner of the data
cell. To view the annotation, right-click the annotated data, and
select ![]() Show annotations from
the context menu.
Show annotations from
the context menu.
In the case of bioMérieux endotoxin detection assay documents, you can find individual columns for initial and final response values.