Execute the OQ
Execute the Operational Qualification to verify the correctness of mathematical calculations performed by PLA 3.0 at your workstation, using datasets verified by Stegmann Systems.
About this task
Each PLA 3.0 data analysis add-on comes equipped with protected operational reference data in the form of an Operational Qualification package (OQ package). An OQ package contains a set of verified datasets in the form of Operational Qualification documents (OQ documents).
When you execute an OQ, an OQ test document is created for each OQ document.

The OQ test document holds the verified dataset of the respective OQ document. It typically checks a single aspect of a complex calculation routine and contains descriptions of test purpose, data setup, and expected results. The OQ test document is calculated and the results are compared with the results from the verified dataset.
You can identify an OQ test document by a purple triangle in the upper right corner
of the document icon ![]() .
.
Procedure
- In the Navigator, select the folder where you want to save the OQ test documents.
-
On the Validation menu, select
 Operational Qualification (OQ)….
Operational Qualification (OQ)….
-
In the Operational Qualification (OQ) dialog, select the
following data:
- Source file: The OQ package of the add-on you want to qualify.
- Report file: The folder where you want to save the OQ report. By default, the OQ process uses the default output path set in the PLA 3.0 preferences.
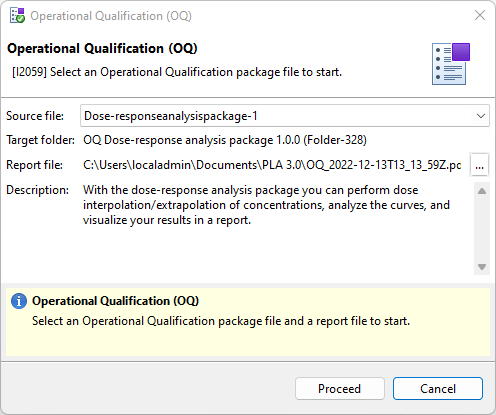
Figure 2. Selection of the OQ setup for the Dose-response analysis package - To start the OQ process, select Proceed.
-
A dialog informs you about the progress and displays a corresponding message
when the process is completed. To directly view the report, select OQ
Report.
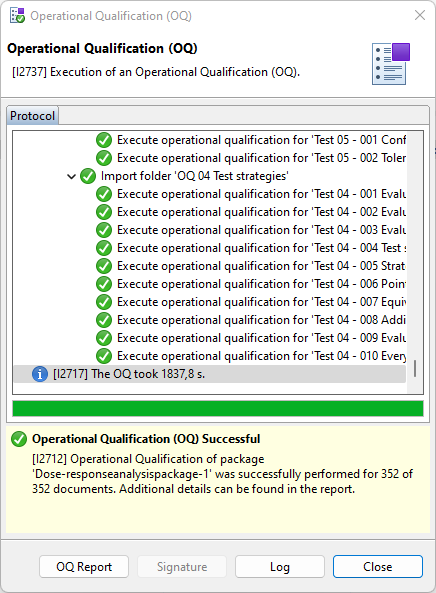
Figure 3. Protocol details of a successfully executed OQ process Tip:To view details about the cryptographic signature applied to a specific protocol entry, select the entry and select Signature.
Results
PLA 3.0 saves the OQ report in the folder you selected. If the execution was successful, the Certificate of Qualification is displayed on the first page of the report. If deviations are detected, the OQ fails and the report is generated without a certificate.
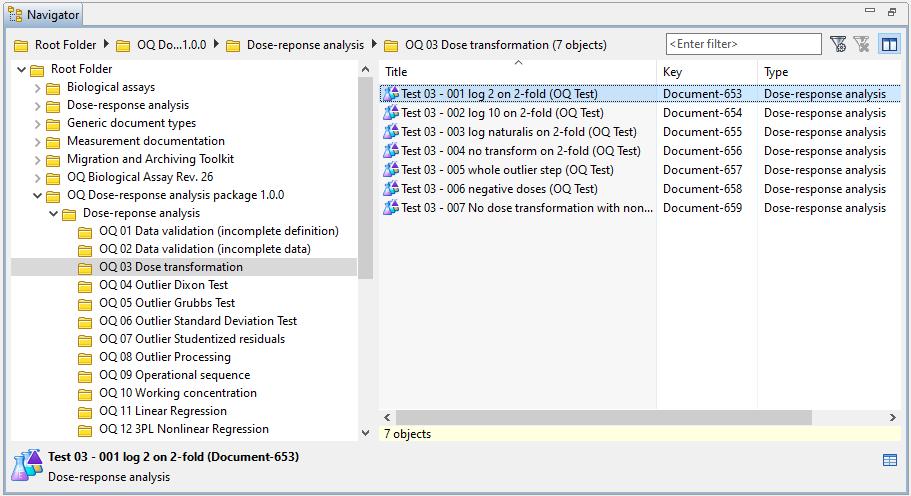
In the Navigator, an OQ folder is created for the validated add-on with subfolders for each document type. The OQ test documents of each document type are displayed in subfolders, mirroring the calculation routines they belong to.
This setup allows for easy extensibility of the OQ data. Additional calculation routines can be added without touching the original folder tree. Verification of OQ test documents within these folders remains valid.
Example
In the following example, an OQ was performed for the Dose-response analysis package version 1.0.0. For the Dose-response analysis document type, the test results for dose transformation are available in the 'OQ 03 Dose transformation' subfolder:
How-to video
The following video shows how you execute an OQ in PLA 3.0:
